CIQUP has consolidated its position as an experimental research unit with strong international recognition in several fields of Chemistry. Below is a selection of what we consider the most relevant contributions to five different topics.
Research Groups
For more information about Research Groups, please click here.
Thermodynamic insights for understanding the stability and reactivity of chemical compounds within the fields of energy, environment, and health
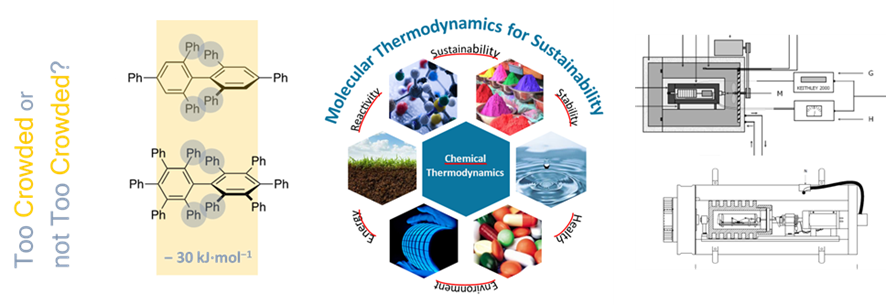
CIQUP maintains its unique capabilities in the field of chemical thermodynamics, which has been internationally recognized. Considerable amount of reliable thermochemical and thermophysical results that are regularly included within the NIST and other databases for reference use by the world scientific community.
Chemical stability of persistent organic pollutants, pesticides, biomass derivatives, along with their mobility-related properties.
Synergies on the trio structure-energy-reactivity of these compounds along with other bioactive substances were established. Experimental and theoretical approach to quantify and understand phenomena of electron correlation in aromatic molecules. Thermodynamic and kinetic study of reactions involving fullerenes. Impact of molecular structure (symmetry, curvature, substitution pattern) on equilibrium and rates of reaction.
Theories, methods, and practices through which the ecologies of education and science communication
The Education, Science Communication, and Society group focuses on theories, methods, and practices through which the ecologies of education and science communication are structured. With a special focus on chemical science, the research group recognizes the ubiquitous and polymorphic nature of digital media as a privileged means of redefining the configuration and comprehensiveness of these ecologies in a global and systemic society. The contextualized and relational explication of chemistry in relation to other disciplinary areas, knowledge, cultural and social contexts, are still an important focus of action and investigation.

Development of new nanomaterials for sensing, energy storage, catalytic and therapeutical applications
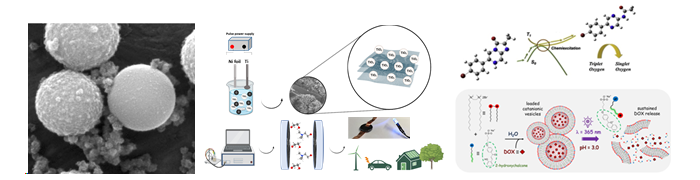
CIQUP team is committed to the development of sequential extraction procedures for toxic metals, new materials with improved performance for energy storage, electro and photochemical degradation of pollutants, and new sensing solutions for screening diseases (e.g. cancer, allergies, bacterial infection).
Novel development of tumor-selective, single-molecule, and self-activating chemiluminescent photosensitizers for broad-scope and enhanced anticancer therapy were achieved.
The interplay of anionic and cationic surfactants led to the creation of novel dual light and pH-responsive catanionic vesicles, for targeted anticancer drug delivery. Additionally, the structural characteristics and properties of thermotropic ionic liquid crystals were mapped across various surfactant families.
Development of advanced analytical and physico-chemical methodologies
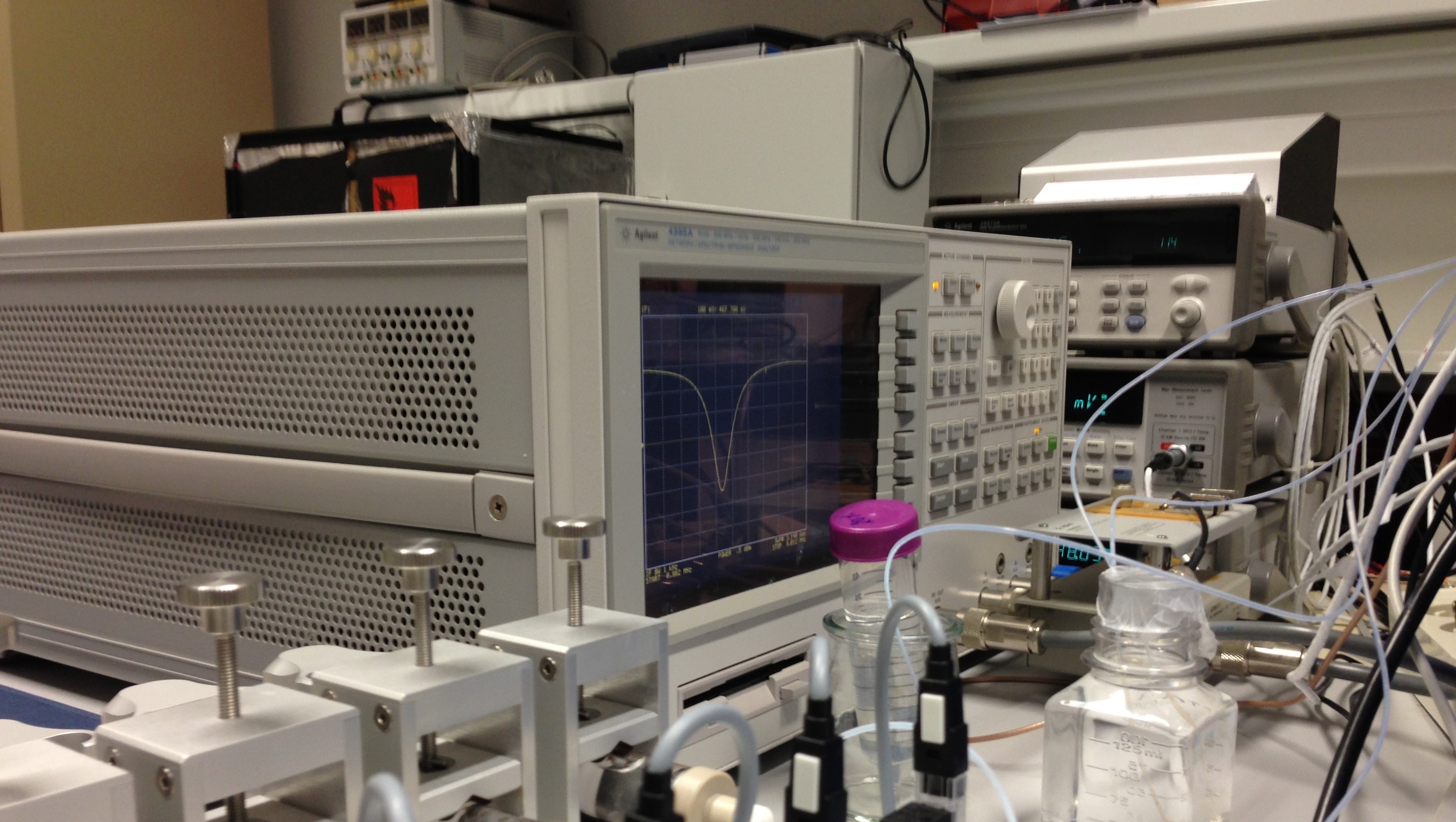
An important hallmark of this unit has been the development of prototype instrumentation and novel experimental methodologies in the fields of analytical and physical chemistry, resulting in advances on the molecular understanding of systems and processes.
A new methodology for the evaluation of the optical band gaps of organic semiconductor materials was introduced as an easy and forthright technique to accurately estimate the band gap energy of organic p-conjugated materials, widely used as thin films/composites in organic and hybrid electronic devices. The development of the above methodology was possible due to our ability to fabricate high quality thin films of organic semiconductor materials driven by our (world class) competence in the field of Knudsen effusion technic based on the QCM (quartz crystal microbalance).
A leading contribution in CIQUP has been also made on chemi- and bioluminescence research. It has been determined that efficient chemiexcitation results from the formation and posterior thermolysis of a dioxetanone intermediate. Our expertise allowed us to tune the chemiexcitation process in the latter reaction to develop new molecules capable of intracellular self-excitation and ROS-production, usable in a new modality of photodynamic cancer therapy.
Advancements in thermodynamics
The merit of the laboratories of CIQUP in the field of chemical thermodynamics has been internationally recognized since long time. These labs produce a considerable amount of reliable thermochemical and thermophysical results that are regularly included within the NIST and other databases for reference use by the world scientific community.
Enthalpies and Gibbs energies of formation of homo- and heterocyclic (with N, O, and S atoms) compounds were determined with high accuracy and used to assess the thermodynamic stability of the compounds, in gaseous and condensed phases, as well as to rationalize the interplay between their energetics, aromaticity and structural properties. Intermolecular hydrogen bond energies (N-H-O, O-H-O and N-H-N) in organic crystals were also experimentally determined.
High-quality predictions of environmental mobility properties of chemical compounds, such as standard Gibbs energy of hydration, vapour pressure, aqueous solubility, Henry constants, and octanol/water partition coefficients, were successfully achieved for halogenated benzenes. The applicability of the equations to the accurate prediction of the properties of benzene derivatives covering other non-ionizable substituents was proven.
Unraveling nanostructures in ionic fluids
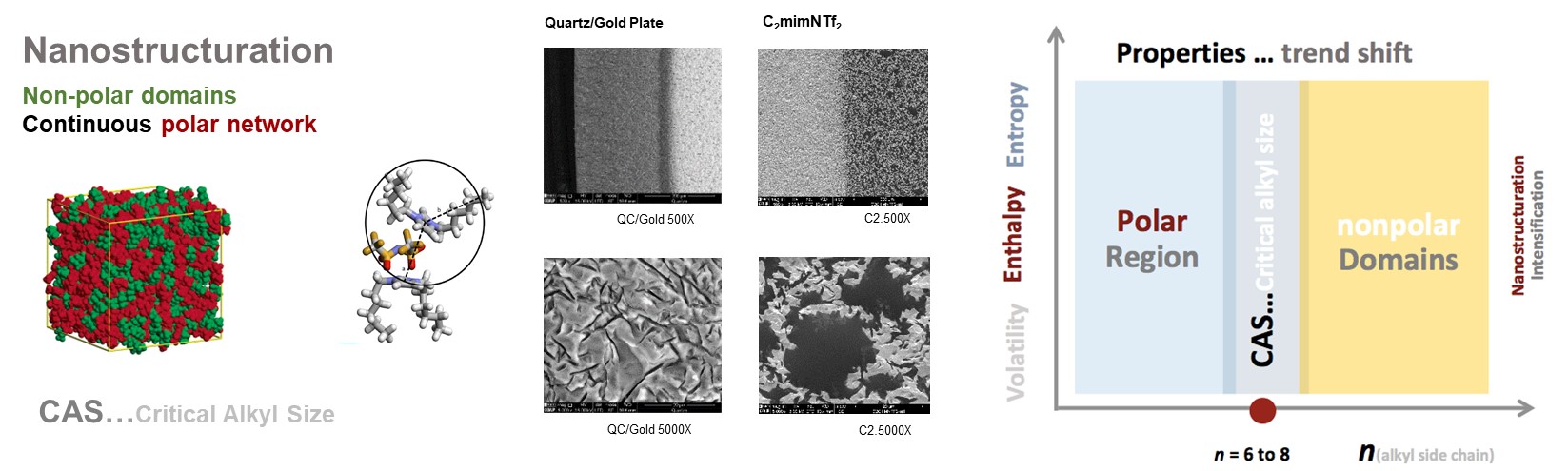
CIQUP has made breakthrough contributions to the experimental study of nanostructuration phenomena in ionic liquids (IL). The key role of alkyl chain length on IL nanostructuration was highlighted, being now widely accepted in the literature that this phenomenon is intensified after the Critical Alkyl Length (CAL) of six carbon atoms. This new model of interpretation of IL has had impact in the scientific community and is now widely used in the rationalization of IL properties and functionality in the bulk and at interfaces. Under this framework, further advances were made on: the impact of cation symmetry on IL nanoscale structuration; self-aggregation of IL; cation alkyl side chain length and symmetry effects on the surface tension of IL; thermal behaviour anomalies.
Measurements of differential capacities for IL and their mixtures revealed interfacial nanostructuration and theoretically predicted phase transitions. A result with potential great impact in the development of double layer supercapacitors was the achievement of double layer capacity enhancement using appropriate IL mixtures.
Understanding structures and functionalities of interfaces
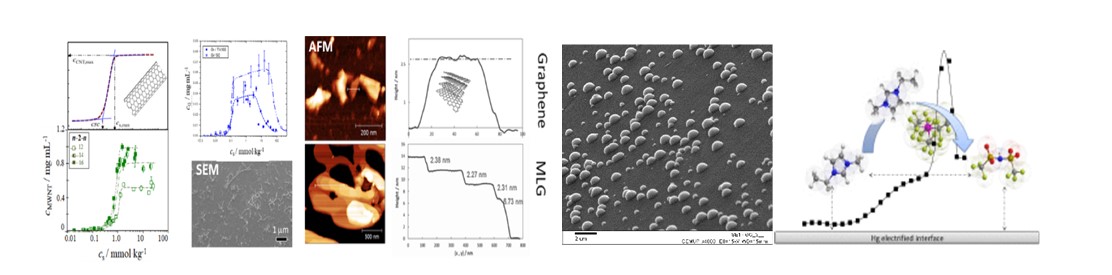
Expertise on both electrified and non-electrified Interfaces (solid/liquid, liquid/liquid and liquid/gas) and on the preparation of advanced architectures of composite materials with controlled thickness were used to obtain key results. These include the development of enhanced sensing devices by controlled growth of hierarchical interfacial structures. In particular, the design and synthesis of new monomers for molecular imprinting maximizing the non covalent bond of target molecules with successful application in achieving very high degree of enantiometric separation. Micro and mesoporous composites with relevant sorption features for metal cations were successfully prepared using materials extracted from fisheries residues. The designed scheme was inspired by biological silicate bridge found on chondrontoin sulfate, offering a potential application in biosorption, sensing and separation processes. Green processes for electrodeposition of metals and alloys using ionic fluids (ILs and deep eutectic solvents) were developed.
High quality data on the dispersion of carbon nanotubes using ionic amphiphiles were obtained and new objective metrics were introduced, a novel approach that allowed us to relate the dispersion process with the molecular properties of the dispersants. This approach may prove crucial to develop non-covalent hierarchical 3D materials in a rational way.
Design, synthesis and screening of bioactive compounds
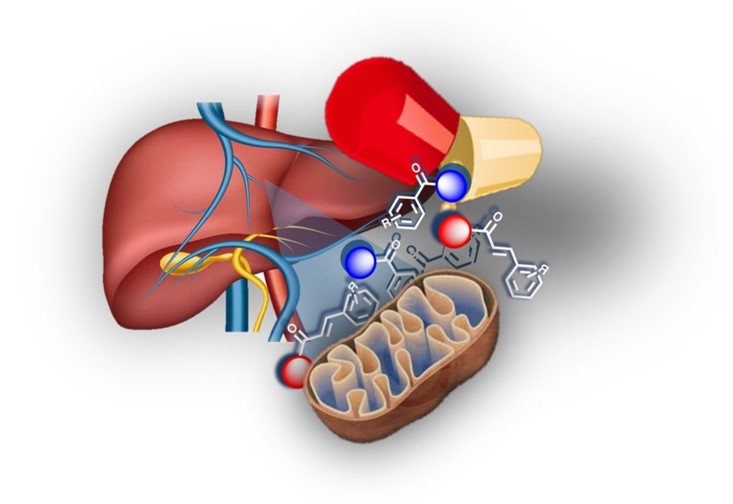
Regarding drug discovery for neurodegenerative diseases, innovative chemical entities based on chromone, cinnamic and benzoic acids scaffolds were obtained. The strategies included: a) rational design and synthesis of concise and diversity-oriented libraries guided by target- and cell-based phenotypic screenings; b) hit-to-lead and lead optimization performed through tailored structural modifications based on (bio)isosterism and guided by structure-activity-relationships; c) pharmacokinetic and pharmacodynamic optimization, including improvement of pharmacological potency and target selectivity, drug-likeness, solubility, membrane permeability and toxicity.
The new entities included: a) a potent, selective and reversible chromone-based MAO-B inhibitor with improved safety and drug-like properties over MAO-B inhibitors used in Parkinson´s; b) the first centrally active and non-hepatotoxic nitrocatechol-based COMT inhibitor, with improved safety over tolcapone, an inhibitor used in clinic; and c) the first mitochondriotropic antioxidants based on a dietary antioxidants with therapeutic potential in mitochondrial oxidative stress-related diseases, like Alzheimer´s and Parkinson´s.
